
Some things were missing during that time and today as students are so comfortable with their studies because everything is available online but before the students have to work themselves and no other resources were present during that time. The periodic table as a list of elements arranged so as to demonstrate trends in their physical and chemical properties.Before in the early 90s, there was not much technology Periodic Table available and because of this many things were affected if we compared today’s technology.Describe and model the structure of the atom in terms of the nucleus, protons, neutrons and electrons comparing mass and charge of protons neutrond and electrons. Use the Periodic Table to predict the ratio of atoms in compounds of two elements. Unit 1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis.Unit C1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis.(g) elements being arranged in order of increasing atomic number and in groups and periods in the modern Periodic Table, with elements having similar properties appearing in the same groups.2.2 ATOMIC STRUCTURE AND THE PERIODIC TABLE.(h) elements being arranged in order of increasing atomic number and in groups and periods in the modern Periodic Table, with elements having similar properties appearing in the same groups.1.2 ATOMIC STRUCTURE AND THE PERIODIC TABLE.Unit 1: CHEMICAL SUBSTANCES, REACTIONS and ESSENTIAL RESOURCES.(a) elements being arranged according to atomic number in the Periodic Table.Unit 1: THE LANGUAGE OF CHEMISTRY, STRUCTURE OF MATTER AND SIMPLE REACTIONS.

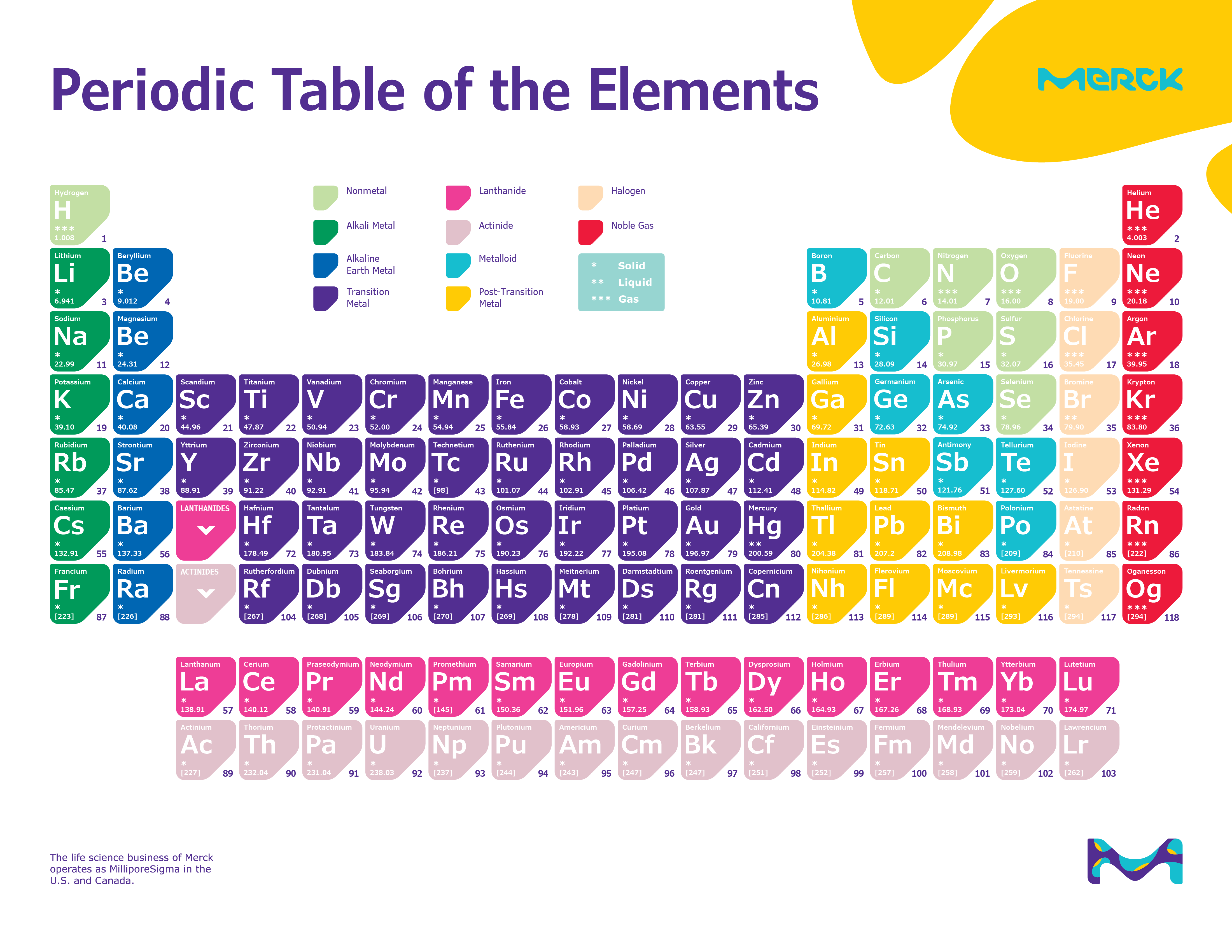
The Periodic Table can be used to determine whether an element is a metal or non-metal.Atomic structure and bonding related to properties of materials.Elements are arranged in the periodic table in order of increasing atomic number.Hamied Inspirational Chemistry Programme.Introductory maths for higher education.The physics of restoration and conservation.


 0 kommentar(er)
0 kommentar(er)
